Editor’s note: Many of you already know Keith Holden, MD, an internist and also a fellow prostate cancer patient, through his Substack Prostate Cancer Secrets. Secrets is a well-written account of Keith’s journey with advanced porostate.
Most of Keith’s readers actually came from TheActiveSurveillor.com. My readership is a Secrets fan and so am I. His is proof that high-risk, low-risk, or inbetween are a “reluctant brotherhood.” I asked him to give to share his thoughts on Active Surveillance. One big point Keith makes: Men on AS resisting biopsies and digital rectal exams can find themselves with advanced prostate cancers. Discuss this with your doctor. Also, be aware that MRIs can play of role in identifying whether you need a biopsy and can make up for not having a DRE.
Keith told me: “I was never on AS. The delay in my diagnosis was due to a protracted symptomatic prostatitis. The urologist didn't want to do a biopsy while I had symptomatic prostatitis.
“I was 52 at the time of onset of symptoms. Diagnosed at 53. I'm 59 now. When I was 52, U.S, Preventive Services Tak Force was not recommending automatic PSAs. I found out just before my biopsy that one of my older brothers was also diagnosed with prostate cancer.
“Prostate biopsy Dec 2017: Gleason was a mixture of 3 + 4 (7) and 4 + 3 (7). April 2018 prostatectomy was 4 + 3 (7) involving 70% of the gland, with 2/9 positive lymph nodes, positive surgical margins, and seminal vesicle invasion. s/p IMRT to pelvis in August 2018 and proton therapy to para-aortic nodes in January 2023. PSMA PET in January 2024 showed no evidence of malignancy despite a PSA of 19.”
By Keith R. Holden, MD
Active surveillance (AS) is the standard of care for monitoring low-grade, low-risk Gleason 6 and favorable intermediate-risk Gleason 7 localized prostate cancer. This approach to low-risk prostate cancer started after physicians noticed a pattern of overtreatment of low-risk prostate cancer, resulting in severe side effects from prostatectomy, radiation, and androgen deprivation therapy.
The goal of AS is to monitor for possible progression while preventing or delaying therapy with inherent side effects. Another goal of AS is curative intent - if the patient progresses and needs treatment, appropriate protocols would catch the progression early enough for treatment to result in a cure.
(Keith R. Holden, MD, and his husband Mike.)
Pioneers of active surveillance
Academic centers like Johns Hopkins, the University of California at San Francisco, and Memorial Sloan Kettering were some of the earliest pioneers of active surveillance in the United States (U.S.). They established studies and protocols in the mid-1990s to generate long-term data that have helped make AS the preferred approach for low-risk prostate cancer that it is today.
AS also has roots in Canada. In 2002, Dr. Laurence Klotz published a prospective active surveillance trial at the University of Toronto, which helped establish its feasibility and safety.
Michigan Urologic Surgery Improvement Collaborative
Capitalizing on the success of these early adopters of AS is the Michigan Urologic Surgery Improvement Collaborative (MUSIC). Established in 2011 with support from Blue Cross Blue Shield of Michigan, MUSIC aims to improve urological care in Michigan.
MUSIC is the only AS program I could find that publishes its protocol in exquisite detail online, calling it the MUSIC AS Roadmap. MUSIC’s AS roadmap closely follows the National Comprehensive Cancer Network's (NCCN) guidelines for active surveillance of prostate cancer.
MUSIC's AS Roadmap splits the protocol into several parts, starting with:
The Consideration Phase: Provides steps to decide whether men are AS candidates.
Step 1: Estimate life expectancy.
Men with a life expectancy of less than ten years enter watchful waiting
Men with a life expectancy of ten years or greater move to Step 2
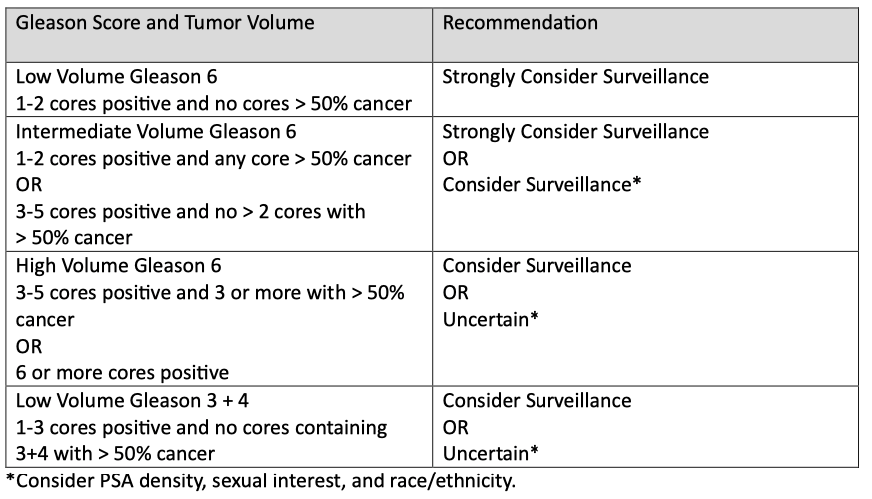
Step 2: Determines appropriateness for AS based on MUSIC criteria and factors in the Gleason score, tumor volume, PSA density (compares PSA level to prostate size), sexual interest, race, and ethnicity.
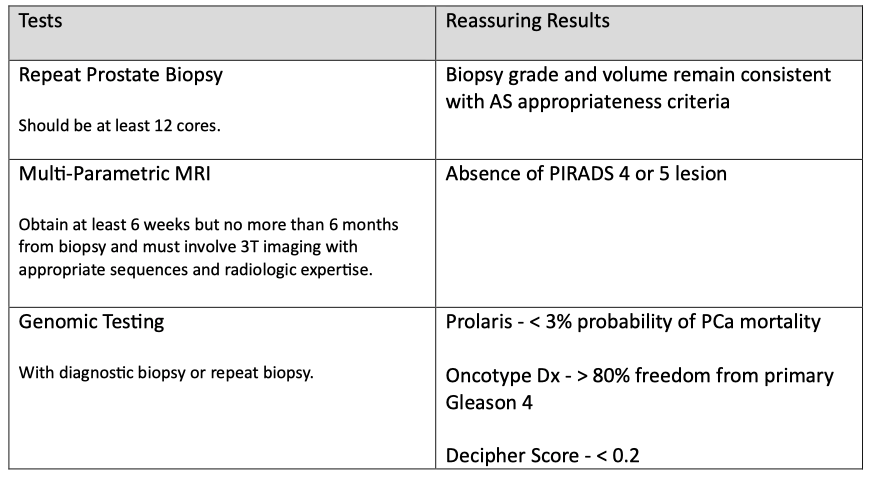
Step 3: Obtain confirmatory testing to evaluate appropriateness.
Sometimes, the initial grade and volume of the tumor are wrong, and this step uses repeat prostate biopsy, multi-parametric magnetic resonance imaging (mpMRI), or genomic testing to increase the confidence that AS is appropriate.
Step 4: Engage in shared decision-making whether or not to begin treatment or enter the surveillance phase.
Once men enter the Surveillance Phase, practitioners and patients choose either a high-intensity or low-intensity surveillance plan, which varies by frequency of planned follow-up.
Situations that would prompt further assessment during AS include:
New and concerning digital rectal exam findings
PSA doubling time < 3 years
Other clinical suspicion for disease progression
Patient preference
For more details about MUSIC's AS program, here is a YouTube video that walks you through the roadmap.
Caveat for Gleason 3 + 4 tumors
I don't see where MUSIC's roadmap factors in cribriform growth patterns in men with low-volume Gleason 3 + 4 tumors, but I suspect it does. A study suggests active surveillance programs should consider cribriform growth patterns when selecting Gleason 3 + 4 prostate cancer patients.
It found that Gleason 3 + 4 tumors with cribriform growth are associated with unfavorable outcomes and metastasis, while Gleason 3 + 4 without cribriform growth showed similar clinical behavior to Gleason 3 + 3.
In addition, an article about prostate cancer prognostic factors published in 2022 opined that the presence of cribriform growth pattern and intraductal carcinoma of the prostate should exclude Gleason 3 + 4 patients from AS.
However, challenges include inconsistencies in defining and reporting cribriform morphology on pathology reports and low detection on prostate biopsies due to sampling issues.
Prevalence of AS
A cohort study published in 2022 reports that U.S. rates of active surveillance increased sharply from 26.5% in 2014 to 60% in 2021."
MUSIC's AS program is likely the most successful in the United States. Howard Wolinsky reports that "it [currently] leads the country in putting low-risk patients on AS with a 91% rate."
The Active Surveillor
By Howard Wolinsky…
a year ago · 3 likes · 4 comments
Europe had a significant head start, with one study showing 85% of men in the Netherlands diagnosed in 2015-2016 with very low-risk prostate cancer were on AS. Another study showed that 91% of Swedish men diagnosed with very low-risk prostate cancer in 2014 were on AS.
Safety of AS
Active surveillance of low-risk prostate cancer, when done correctly, is very safe. Since 2015, several prospective protocol-managed AS cohort studies have published their long-term results showing 10- to 15-year metastasis-free and prostate cancer-specific survival rates ranging from 95–100% for low-risk prostate cancer.
Active Care
Prostate cancer centers of excellence use protocols that mostly follow NCCN guidelines. However, as these are guidelines and not rules, these centers are free to tweak their protocols to benefit both practitioners and patients.
For example, Johns Hopkins developed a software tool called Active Care to help prostate cancer patients on AS better understand their condition and help physicians make personalized care decisions.
The Active Care software tool:
Uses a prediction model developed by Johns Hopkins researchers based on decades of active surveillance data
Displays personalized predictions with clear data visualizations
Charts a patient's past lab results, MRI values, and the likelihood of finding a higher grade cancer on biopsy
Shows the probability of each cancer grade and long-term outcomes should a patient have his prostate removed
Helps patients better understand their risks and enables more confident decision-making about remaining in the active surveillance program vs pursuing treatment
Johns Hopkins says that since they deployed this software in 2016, AS team members have used it to make data-driven decisions regarding whether a patient needs to undergo a biopsy or seek treatment.
"Previously, patients were recommended to get a biopsy annually, but with the tool, most patients can wait, sometimes years longer, before undergoing the procedure."
Two significant issues in AS
The fact that patients can safely wait years before another prostate biopsy is emerging as a critical topic in AS. Men do not want digital rectal exams or prostate biopsies, and some will drop out of AS for those reasons, only to show up years later with advanced prostate cancer.
So, as testing and AS guidelines evolve, physicians and researchers should create and use evidence-based software tools like Active Care to decrease the amount of digital rectal exams and prostate biopsies that men undergo while on AS. In addition, optimizing artificial intelligence in AS programs will likely lead to safer and more effective programs with higher retention rates.
Overdetection
Researchers and practitioners should continuously reassess AS guidelines and protocols to find more ways to avoid overdetection of low-grade, low-risk prostate cancers that won't progress. As I discussed in a prior newsletter, Prostate Cancer Screening Part 2 - 016, overdiagnosis, also known as overdetection, is a big problem.
As more evidence-based tests emerge to augment prostate-specific antigen (PSA) testing, practitioners can use them to avoid unnecessary prostate biopsies. As Eric Kim, M.D., a urologist at the University of Nevada, Reno School of Medicine, states,
"...the ideal thing would be to have a non-invasive tool tell you as much as you can, and then you could really restrict the biopsies to the people that you think would benefit from knowing that they have prostate cancer."
Dr. Kim studies AI in prostate cancer and says that this ideal non-invasive tool will likely combine blood, urine, imaging, and AI to tell you who needs a biopsy and who doesn't.
Unnecessary rectal exams
As Howard Wolinsky, the writer of this Active Surveillor substack newsletter, has reported before, there is evidence coming from countries outside the U.S. that digital rectal exams aren't able to detect early prostate cancers and may be unnecessary.
Movember is an Australian men's health charity that funds prostate cancer research. It commissioned a report to gauge expert consensus on best practices and research priorities in active surveillance, which was published online in European Urology Oncology.
The consensus "agreed that best practice includes the use of high-quality magnetic resonance imaging, which can allow digital rectal examination and some biopsies to be omitted."
The concept of omitting unnecessary digital rectal exams and prostate biopsies is groundbreaking and hopefully foreshadows future expert guidelines in the U.S. This would not only help avoid overdiagnosis but would improve patient retention in AS programs.
Expert discussion
In 2020, urologist Matt Cooperberg, M.D. of the UCSF Comprehensive Cancer Care Center, interviewed two pioneers in active surveillance for prostate cancer - Peter Carroll, M.D., prior Chair of Urology at UCSF, and Laurie Klotz, M.D., Chief of Urology at the Sunnybrook institute at the University of Toronto.
Both interviewees said that their interest in active surveillance began after seeing a recurring pattern of overdetection and overtreatment of low-grade prostate cancer. Both agreed that active surveillance for men with Gleason 3 + 3 = 6 is a no-brainer.
However, caution should be taken in men with Gleason 3 + 4 = 7 to ensure that the pathologist's grading is accurate and that pattern four has a low volume. Dr. Carroll says, "It's actually volume more than grade that predicts outcome for 3 + 4 patients…" and recommends MRI and genomic testing to interrogate those with more volume on biopsies.
Dr. Klotz noted that before MRI and biomarkers, he observed that of the one hundred twenty 3 + 4 men he had been following on active surveillance over fifteen years, 30% developed metastases. "Way too high," he said.
He says surveillance for 3 plus small amounts of 4 is reasonable but recommends these men be checked with genomic testing and MRI if they are to remain under active surveillance. He says that while accurate imaging, including micro-ultrasound, makes a considerable difference, imaging is imperfect and misses significant cancers.
Klotz also says he doesn't promote genetic testing for men with very low-risk to low-risk disease, especially if they have favorable imaging and favorable targeted biopsy results. He feels genetic testing is not cost-effective in those men.
Dr. Carroll says that biomarkers appear to be better predictors than MRI for multivariable analyses but that no biomarker absolutely predicts or excludes progression. He recommends that these studies be used in context to consider an earlier confirmatory biopsy.
Dr. Coppersberg mentions that data from the Michigan Urologic Surgery Improvement Collaborative (MUSIC) and elsewhere show that many men initiated into active surveillance don't complete the minimal requirements.
Dr. Carroll says that to avoid this scenario, "We clearly need to make surveillance less intense for patients," and refers to reducing the frequency of biopsies.
Dr. Caroll mentions that good predictors of nonprogression include:
low PSA density
low PI-RADS scores
favorable genomic scores
a negative biopsy - up to 20% of men on active surveillance will have a follow-up negative prostate biopsy
He says these are factors to consider when following men on active surveillance less intensely.
Dr. Klotz agrees and says that MRI is replacing some of the biopsies and that, in his program, "We're now at about a five-year biopsy interval for the average patient, and I think you have to individualize this."
Dr. Copperberg asks if they think we'll ever get to a point where a low-volume single-core biopsy showing 3 +3 will be labeled something other than cancer.
Dr. Carroll replies, "I don't think so, actually. We've shown that even some people who have repeated negative biopsies can progress in the future. I think the public has to understand that cancer is a disease of increments…"
Dr. Klotz says, "…the nomenclature lies with the pathologist, so they're the ones we have to convince…" He says he's talked to people all over the world, and they're not ready to abandon the word cancer.
The future
Active surveillance of prostate cancer is evolving as researchers longitudinally study databases involving men on AS, such as Johns Hopkins' database, using their Active Care software tool. The technology is proprietary, but they should share the clinical findings with other researchers to create best practices for AS programs worldwide.
Databases containing patient characteristics, biomarkers, pathology slides, and imaging studies combined with artificial intelligence will create AS protocols that safely reduce overtreatment and optimize retention rates.
Creating programs that involve primary care providers and community-based urologists will help ensure prostate cancer patients on AS receive the recommended follow-up testing. Providing adequate education to patients and practitioners is critical in supporting adherence to AS protocols.
In addition, men with prostate cancer and their loved ones need adequate psychological support so they can make informed decisions in a supportive environment. The psychological burden of AS and fear of rectal exams and prostate biopsies is something practitioners need to factor in each time they interact with patients on AS.
A good motto for this might be, "Put yourself in their shoes." I think that's what the 2023 Movember consensus panel did when deciding that the use of high-quality MRI can safely reduce rectal exams and prostate biopsies for men on AS.
I can guarantee that the U.S. will lag other developed countries in this regard. Why? Because we've already seen this pattern in the adoption of AS compared to European countries.
Incentives are necessary
Why did MUSIC's AS program take the lead in adopting AS in the U.S.? One reason is a financial incentive. As reported in the Urology Times Journal,
"When the collaborative meets specific performance targets, participating urologists receive higher reimbursement (up to 105% of standard fee) from Blue Cross Blue Shield of Michigan (BCBSM)."
Doctors, for the most part, have the patients’ best interests at heart. But what sustains the incentive to always do the right thing? Higher reimbursement rates and feedback on meeting AS practice parameters.
It's too bad we can't create that kind of collaboration nationwide because it's already proven to work in one state. If Michigan can do it, so can everyone else.
I wish all of you the very best.
Keith R. Holden, MD, graduated from Louisiana State University School of Medicine in New Orleans, LA, in 1992 as an Alpha Omega Alpha Honor Medical Society member. He completed an Internal Medicine residency in 1995 and is board-certified in Internal Medicine.
He has lectured at numerous medical conferences in the U.S. and Canada on topics including mindfulness, meditation, the placebo effect, the impaired intestinal barrier's role in systemic illness, and the medical benefits of pulsed electromagnetic field therapy.
He has written a book titled “Power of the Mind in Health and Healing,” and his Udemy.com course, which is the same name, ranks in the top 3% of all Udemy courses, with over 21,000 students worldwide.
He was diagnosed with advanced prostate cancer in 2018. Unable to tolerate androgen deprivation therapy, he writes a Substack newsletter, “Prostate Cancer Secrets,” chronicling his experiences as a patient in the American medical system.
Why does he call his newsletter Prostate Cancer Secrets? “‘Secrets; came about when I was going through the medical system as a prostate cancer patient and started on ADT (androgen deprivation therapy) but not told about side effects. And not one doctor ever said that if you stay on ADT long enough, the physiologic pressures put on the cancer cells by the therapy cause mutations resulting in a more aggressive castration resistant tumor and even causing neuroendocrine tumors.
I thought a lot of these doctors aren't telling prostate cancer patients the truth about everything. Thus...secrets.”
The whole prostate cancer world is filled with such “secrets.”
Still time to share your opinions about AI and prostate cancer through Sept. 1
By Howard Wolinsky
Tick-tock, Less than one month to share your opinions on an important issue for urology patients—the growing use of artificial intelligence (AI) in diagnosing and making tumor management choices for prostate cancer,
Around 300 or so of you have sounded off. Let’s shoot for 500. It takes about 15 minutes.
Peter Evancho, an attorney and second-year medical student at the University of Maryland, Baltimore, is conducting a policy analysis about AI and urology and is asking for our help.
Can you take a few minutes and answer Evancho’s survey and share your thoughts about AI and urology care? Survey link: https://rs.igs.umaryland.edu/surveys/?s=3R37KJMPERYEWMH9
The survey is completely anonymous.
The University of Maryland, Baltimore’s Institutional Review Board has approved this study under HP-00109759
PCRI in-person is back, baby
The Prostate Cancer Research Institute’s 2024 Prostate Cancer Patients & Caregivers In-Person will be held in-person for the first time since 2019.
The popular p[atient-oriented meeting switched to virtual because of the COVID-19 pandemic. But it’s back in person, Sept. 7-8 at the Westin Los Angeles Airport.
Details to come. I’ve been invited to moderate the meeting. So say yo.
Register here.
Who’s got short shorts? ASPI does.
Active Surveillance Patients International has carved up episodes of the popular AS 101 series, co-sponsored by ASPI, AnCan Foundation, the Walnut Foundation, and Canada’s Nationwide AS Support Group under the auspices of Prostate Cancer Foundation Canada.
Check out the bite-sized videos edited and compiled by ASPI Executive Director Bill Manning from the Active Surveillance 101 series: https://aspatients.org/a-s-101/ To see the full videos covering PSAs, second opinions, genomics, exercise, and more, go to: https://aspatients.org/a-s-101/
Please weigh in on U.S. Senate funding of CDC research on low-risk PCa
By Howard Wolinsky
The House Appropriations Committee has made significant cuts for Fiscal Year 2025 to the Centers for Disease Control and Prevention (CDC), nearly a quarter of its funding.
Among other things, the CDC supports a number of prostate cancer projects, including outreach into high-risk communities and support for state and local prostate cancer programs, all of which are at-risk for large cuts.
I have some skin in the game. I helped win a $1 million grant from CDC—the first of its kind on minorities—to study opinions about Gleason 6 (Grade Group 1) in African American and Hispanic men. Speak up for my project—probably the biggest study on AS in minority funded by the U.S. government. Use your AS superpowers and let your Senators know about this. (I’ve done it already myself.)
ZERO Prostate Cancer said: “Your elected officials need to hear from you - these programs are critical for improving prostate cancer outcomes and saving lives.
”The Senate is next to weigh in on funding for CDC. We can help protect prostate cancer funding by telling Senators that cuts would be devastating for our community.”
Please check in here: https://p2a.co/15HnJhg



Thanks, Ira.
And back at you at Prostate Forum of Orange County. We're all in this together,
Howard
Thanks, Geoff.
Thanks for what you do at ASPI for this scrappy community.
Howard